The risk of getting a false negative result is relatively high with rapid tests. A list of all COVID-19 rapid antigen self-tests home use tests that are approved for supply in Australia is available on the TGA website along with the manufacturers instructions for how to use the tests.

What Is The Diagnostic Accuracy Of Antibody Tests For The Detection Of Infection With The Covid 19 Virus Cochrane
Under those conditions a rapid test produces correct results 80.
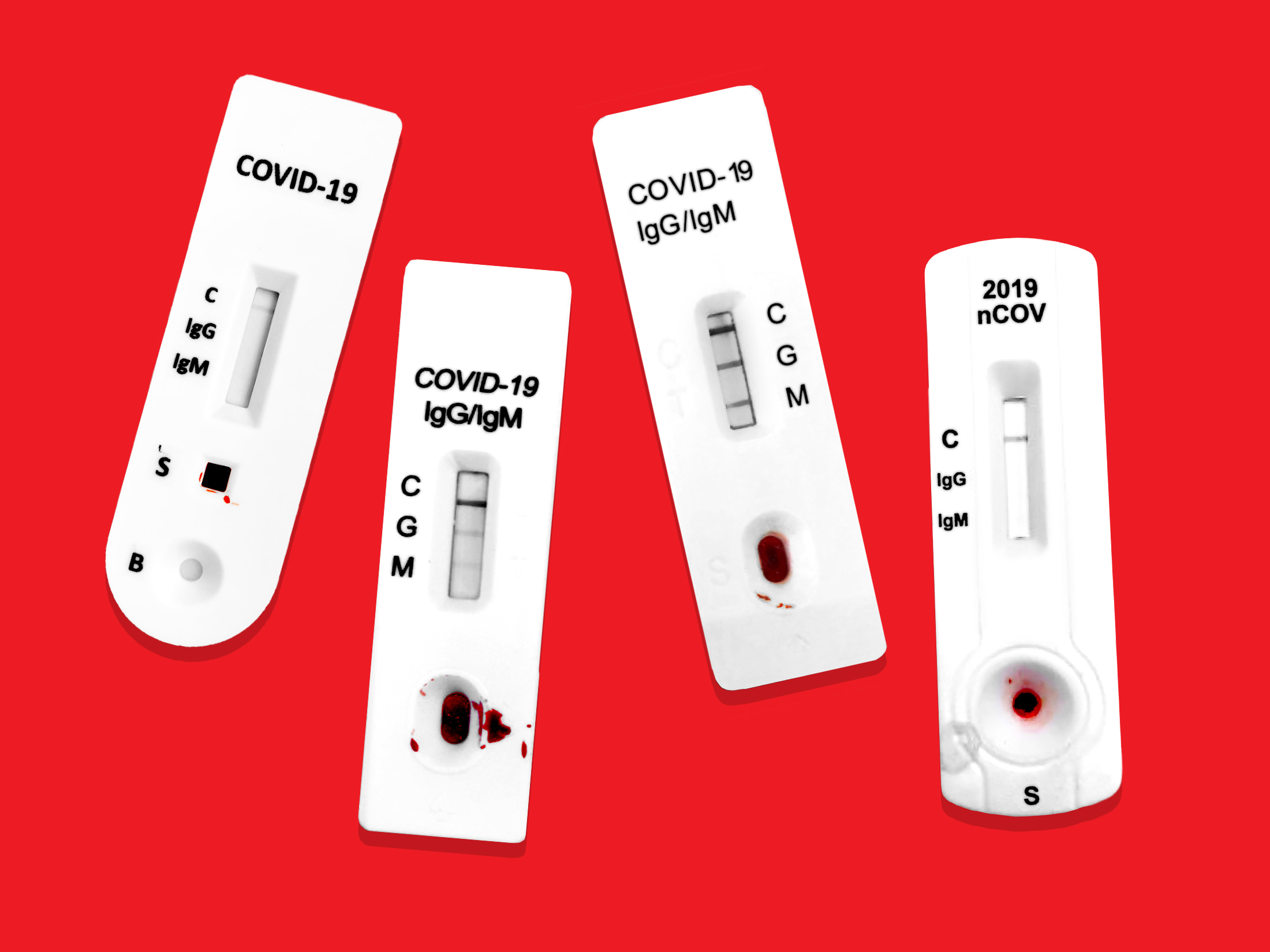
How accurate are rapid covid 19 antibody tests. Based on these data and assuming a 10 prevalence of previous COVID-19 infection the positive predictive value of the AbC-19 test would be 82 meaning that only 4 out of 5 people who recovered from COVID-19 would be correctly classified. Someone with a positive test by this style of test should be treated as infected with COVID-19 but a negative test is less reliable and may need to be confirmed by a more sensitive molecular assay. A positive antibody test indicates a person has antibodies for COVID-19 as a result of.
How accurate is a Covid antibody test. Rapid tests are most accurate when used by people with COVID-19 symptoms in places with a lot of community spread. By ET editorial staff.
Vargas says theres a higher probability of a false-negative test result. If the antibodies are able to neutralize variants of COVID-19 spike proteins printed on the slide they cannot attach to the. So rapid antigen tests can frequently give negative results even if you really have COVID-19.
If you develop symptoms or are identified as a close or casual contact of someone who has COVID-19 NSW Health recommends that you get tested at a clinic and self-isolate. Our findings come mainly from 38 studies that provided results based on the time since people first noticed symptoms. COVID-19 IgGIgM Rapid Test Cassette Manufacturer.
Rapid antigen tests will detect most cases of COVID-19 but are not as accurate as the standard test PCR. An antibody test may not show if you have a current infection because it can take 1 to 3 weeks after the infection for your body to make antibodies. In other words its a pretty accurate test.
Researchers have created a rapid test that they say can identify antibody effectiveness against Covid-19 variants. A prototype device can tell with 95 per cent accuracy if someone has a virus - a significant improvement over current rapid tests - according to the research team behind the idea from the University of Central Florida. Antibody tests one week after first symptoms only detected 30 of people who had COVID-19.
In case of a laboratory test a medical expert collects the blood sample from your vein and gets it at the pathology lab to examine the sample in a proper manner. The new test of how well antibodies work against multiple variants of COVID-19 works by releasing fluorescently labeled ACE2 proteins the cellular targets of the COVID-19 spike protein together with antibodies. For example the COVID-19 Testing Project showed that overall sensitivity of all validated rapid tests reached 80 Se only at 20 days of symptom onset maintaining 95 Sp.
Artron Laboratories Antibody tested. Rapid and highly accurate test detects viruses such as Covid-19. Diagnostic kit IgMIgG of Novel Coronavirus COVID-19.
Rapid COVID-19 Antibody Test Is Not as Accurate as We Were Told Scientists Warn. COVID-19 antibody tests can help identify people who may have been infected with the SARS-CoV-2 virus or have recovered from a COVID-19 infection. Accuracy increased in week 2 with 70 detected and was highest in week 3 more than 90 detected.
Because this process takes a few days they are very likely to give a false negative result in the first few days of infection. Published Tuesday November 30 2021. They found that people who had COVID including.
If you dont have symptoms Dr. Research suggests rapid COVID-19 tests are most accurate when used in the first week after symptoms start. Rapid test identifies antibody effectiveness against COVID-19 variants.
Researchers analyzed antibody tests conducted on more than 500 subjects in patient care settings. The test can quickly and easily assess how well someones antibodies fight. The FDA says antibody tests should not be used to diagnose an active COVID-19 infection because antibodies can take several days or weeks to develop after you have an infection.
In some cases you may still need to get a PCR test. New findings from a Michigan Medicine study reveal that antibody testing is predictive of prior COVID-19 infection and rapid screening methods even from finger pricks are effective testing tools. None of the tests showed 80 Se at 610 days of symptom onset and only half.
Antibody tests external icon should generally not be used to diagnose current infection. One Step Novel Coronavirus COVID-19 IgMIgG Antibody Test Manufacturer. For example the FDA- and CE European Union-approved antibody test from Cellex promises 94 sensitivity percentage of correctly identified true positives and 96 specificity percentage of correctly identified true negatives.
A rapid finger-prick test designed to show whether a person has previously been infected with SARS-CoV-2 is significantly less accurate than earlier research suggested scientists report in a new study. According to the FDA those tests can be less accurate especially when it comes to detecting early COVID infection. Past infection with SARS-CoV-2 or.
Of the tests that performed poorly two tests had close to 0 sensitivity. This list is regularly updated as new tests are approved or if tests are cancelled or withdrawn. A new test can quickly test the ability of antibodies to neutralize spike proteins from different variants of COVID-19.
Antibody serology tests for COVID-19 look for the antibodies produced by the body in response to the virus. As a matter of fact there are two specific kinds of antibody tests and both of these tests can help to determine the presence of antibodies in a person. The rapid molecular-style tests can be reliable if a quality specimen is used and if the testing is conducted by properly trained individuals who are performing the test as intended by the manufacturer.
Specifically many antibody tests have a high false-negative rate and a high risk of bias for participant selection application of index tests reference standard used and flow and timing for antibody tests that may incorrectly report the accuracy of COVID-19 antibody tests.
Accuracy Of Covid 19 Antibody Tests Questioned
A Reality Check On Antibody Testing How Do We Race Forward Thoughtfully Abc News
Coronavirus Tests Are Pretty Accurate But Far From Perfect

Are We Failing The Coronavirus Antibody Test The New Yorker

Coronavirus Antibody Tests May Produce Contradictory Results Bloomberg

Tidak ada komentar:
Posting Komentar