But the validation tests these companies have performed varied widely in size. Abbotts antibody assay was tested on 1200 specimens whereas Epitopes tests were run on 54 samples from healthy people and just 20 and 30 cases of PCR-confirmed Covid-19 for the IgM.
Panbio Covid 19 Igg Igm Rapid Test Point Of Care Abbott
COVID-19 antibody tests can help identify people who may have been infected with the SARS-CoV-2 virus or have recovered from a COVID-19 infection.

How accurate is the abbott antibody test for covid-19. PCR tests are generally more accurate than rapid tests. Covid-19 Antibody Blood Test from 29th March 2021 we have been using the Roche spike protein combined IgGIgM test For those aged 12 and over. The tests authorized by the FDA are safe and effective but no test is 100 accurate and when dealing with a disease with relatively low prevalence such as Covid-19 there will statistically be more false positives.
Short clinic visit by appointment. Similarly Abbotts AdviseDx SARS-CoV-2 IgM antibody test has a 9956 specificity and 95 sensitivity for patients tested 15 days after symptoms started. FDA Cautions About Accuracy Of Widely Used Abbott Coronavirus Test.
If a person gets tested for antibodies after receiving a vaccine they might test positive by some but not all antibody tests. This test has a sensitivity of 100 meaning the test will currently identify COVID-19 IgG antibody if it is present in the blood 100 of the time and a specificity of 998 meaning the test will correctly determine that. Most of these antibody tests have 95 sensitivity meaning that about 95 of people with antibodies will correctly receive a.
As of October 2021 there are nearly 100 different antibody tests that are authorized by the US Food and Drug Administration FDA. Most coronavirus antibody tests focus on these two antibodies as opposed to IgA which is found mainly in the respiratory and digestive tracts. Its also already being rolled out in some US.
Five at-home over-the-counter COVID-19 tests have received FDA authorization. The tests do not work accurately. This is called the specificity of the test.
Currently the ID NOW COVID-19 test is available only in the US. The comparison which relies on data from the companies shows variation in the ability of serology assets to reliably determine if a sample contains antibodies against the pandemic coronavirus. The Healgen test for IgG antibodies had 967.
CT scans are rarely used to diagnose COVID-19. Abbott was not the first company to get emergency use authorization for a point-of-care coronavirus test Cepheid claimed that milestone. If you have symptoms the test is accurate in telling you if they are the result of COVID-19.
COVID-19 antibody tests from Roche and Siemens scored highly on a comparison of emergency authorized tests published by FDA. Were also supplying our m 2000 tests in the US and are shipping as many tests as possible to customers around the world. Food and Drug.
T his week the European Union approved an antibody test for Covid-19 that its manufacturer says is 99 accurate. Small blood sample taken from your arm. Antibody tests are very accurate at detecting whether antibodies are present or not.
COVID-19 antibody tests look for evidence of past exposure to the SARS-CoV-2 virus not an active infection. A lot has changed since the early days of the pandemic when. Another notable caveat regarding Covid-19 antibody tests is testing accuracy.
Abbott said that the test had demonstrated specificity and sensitivity of greater than 99. As this is where the majority of our ID NOW instruments are in use today. The testwhich has received its CE mark meaning that it complies with EU safety rulesdetects the antibody IgG to identify whether a person has had covid-19.
The Abbott test also tells you that the antibodies the test detected are antibodies to the COVID-19 virus 9963 of the time. In a recent post we warned readers against rushing out to purchase one of the many COVID-19 antibody tests suddenly flooding the market noting that few of these tests have been independently validated and many are grossly inaccurate. But what about the tests the US.
Produced by the global diagnostics company Abbott the test received a CE mark signifying that it meets the EUs safety standards. Heres a rundown of how accurate each test is compared to PCR testing. MSU study looks at the two COVID tests Two types of tests detect COVID-19.
An antibody blood test for covid-19 which the manufacturer Abbott claims is 99 accurate has been certified for use by the European Union. Cities after receiving Emergency Use Authorization from the US. Antibody tests can be used to diagnose past infection.
But the potential for Abbotts test to detect positive cases in five minutes and negative results in 13 minutes as well as the companys claim its ID Now machines were the most widely available molecular POC testing platform in the country. The table above summarises some of the accuracy figures that selected manufacturers claim for their serological Covid-19 tests. Three are rapid antigen tests from Abbott Ellume and Quidel and the other two are molecular-based tests from Cue Health and Lucira.
But because of the wide availability of COVID-19 vaccines the topic of testingand which one to usehas fallen off our collective radar. Scientists have analysed data from around the world to examine the accuracy of antibody tests for COVID-19 and have shown that the timing of testing is critical. A handful of rapid at-home tests are available without a prescription including the Abbott BinaxNOW the Ellume Covid-19 Home Test and the Quidel QuickVue At-Home Covid-19 Test.
A venous blood draw tends to be more accurate but a finger-stick test yields quicker results. Coronavirus Updates The test has been promoted by the Trump administration as a key factor in controlling the epidemic in the U. Result e-mailed to you within 3 working days for samples taken on Fridays after 1pm.
Most tests cant detect antibodies until 11 to 18 days after symptom onset or virus exposure. With kids returning to school and many adults heading back to the office or traveling something as simple as a cough or runny nose might lead to panicand the need for a COVID-19 test. Tests with FDA Emergency Use Authorization have varying degrees of accuracy.
Antibody testing is not currently recommended to assess for immunity to COVID-19 following vaccination. Antibody tests rely on blood samples. The COVID-19 antibody test we use at Nuffield Health is very accurate and its been approved by Public Health England.

Covid 19 Antigen Testing Diagnostics Testing Newsroom

Panbio Covid 19 Igg Igm Rapid Test Point Of Care Abbott

Covid 19 Antibody Tests Aren T A Magic Bullet To Escape Lockdown
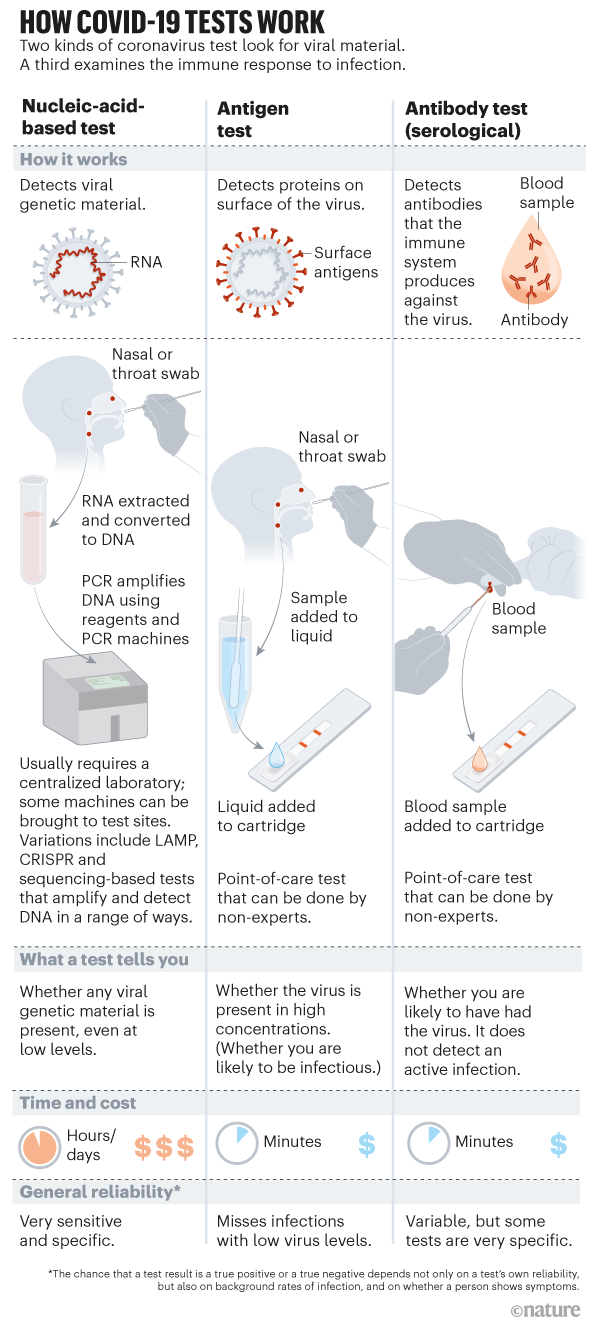
Rapid Coronavirus Tests A Guide For The Perplexed
Abbott Our Newest Test Can Detect Antibodies To The Novel Coronavirus Learn More About Antibodies And Why It S Important To Know If You Have Them Facebook


Tidak ada komentar:
Posting Komentar